Unlocking The Secrets Of Methane's Lewis Dot Structure
Hey there, science enthusiasts! If you're diving into the world of chemistry, understanding the Methane Lewis Dot structure is like having a secret decoder ring. This diagram shows how valence electrons are arranged around atoms, giving us a peek into molecular bonding. Whether you're a student, a researcher, or just someone curious about the basics of chemistry, this guide will take you deep into methane's Lewis dot structure and why it matters so much.
Let’s talk about methane for a moment—it’s one of the simplest hydrocarbons out there, but don’t let its simplicity fool you. It plays a huge role in both organic chemistry and environmental science. With the molecular formula CH₄, methane consists of one carbon atom bonded to four hydrogen atoms. The Lewis dot structure is like a map that helps us understand how these atoms bond together, making it easier to grasp methane's properties and behavior in different situations.
In this article, we’re going deep into the Methane Lewis Dot structure, exploring its practical applications, and highlighting why it’s such a big deal in chemistry. Whether you're new to the subject or looking to deepen your knowledge, we’ve got you covered. We'll break down methane's electron distribution and molecular structure in a way that’s engaging and easy to understand. Stick with me, and let's uncover the science behind methane!
Read also:Understanding The Anna Malygon Leak A Deep Dive
Inside the Methane Lewis Dot Structure
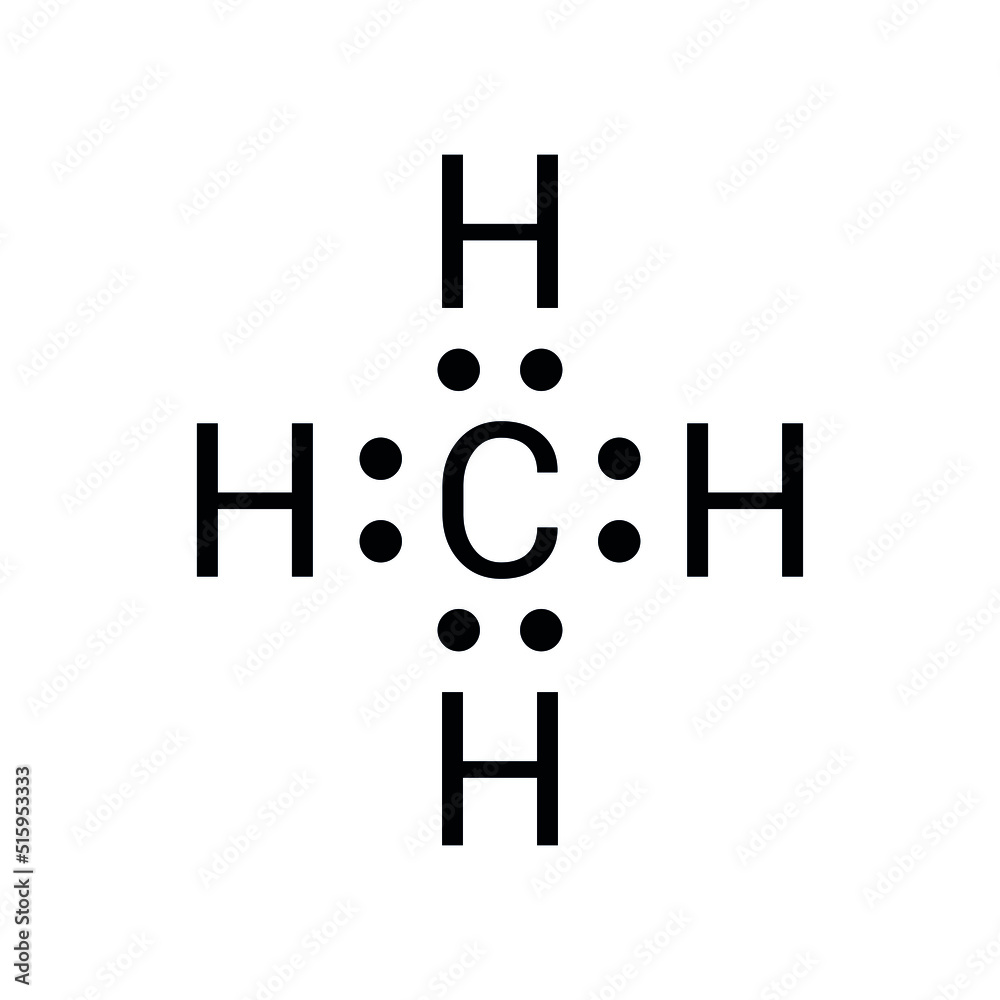
The Methane Lewis Dot structure is basically a visual tool that shows how valence electrons are spread out among the atoms in a molecule. For methane (CH₄), this structure highlights the single covalent bonds formed between one carbon atom and four hydrogen atoms. Each bond consists of two shared electrons, ensuring that all atoms have a stable electron configuration. This stability is key to keeping the molecule intact and functioning properly.
Now, methane’s Lewis dot structure is pretty straightforward. Carbon brings four valence electrons to the table, and each hydrogen atom chips in with one. Together, they create a tetrahedral shape, which is crucial for understanding methane's geometry and how reactive it is. This geometric arrangement not only defines the molecule's physical properties but also affects how it interacts with other substances. Think of it like a blueprint for methane's personality in the chemical world.
Why Dive Into Methane Lewis Dot?
- To really get a handle on molecular bonding and how electrons are distributed.
- To accurately predict the shape and properties of methane molecules.
- To use this foundational knowledge in fields like organic chemistry, environmental science, and beyond.
Breaking Down Methane's Chemical Makeup
Methane (CH₄) is made up of one carbon atom and four hydrogen atoms. Carbon, which sits in Group 14 of the periodic table, has four valence electrons. Hydrogen, in Group 1, contributes one valence electron per atom. Through covalent bonding, these atoms share electrons equally, forming a stable molecule. This stability is thanks to the octet rule, which says that atoms like to gain, lose, or share electrons to end up with a full outer shell of eight electrons. In methane's case, carbon achieves this by forming four covalent bonds with hydrogen atoms, creating a balanced and stable structure.
The stability of methane is a great example of how effective covalent bonding can be. The shared electrons create a strong, cohesive molecular structure. This is one of the reasons methane is so widely used in various industries, from producing energy to manufacturing chemicals. It’s like the rockstar of the chemical world!
Key Features of Methane
- Molecular formula: CH₄
- Valence electrons: 8 (4 from carbon + 1 from each hydrogen)
- Bond type: Covalent
- Molecular geometry: Tetrahedral
How to Draw Methane’s Lewis Dot Structure
Drawing the Methane Lewis Dot structure is a step-by-step process that requires a systematic and methodical approach. Follow these steps to create an accurate representation of the molecule:
- Count those valence electrons: Carbon contributes 4 electrons, and each hydrogen contributes 1, adding up to a total of 8 electrons.
- Position the atoms: Place the carbon atom at the center and surround it with four hydrogen atoms.
- Form single covalent bonds: Connect carbon to each hydrogen atom by sharing two electrons per bond.
- Check for stability: Make sure all atoms have a stable electron configuration, with carbon achieving an octet and each hydrogen having two electrons.
Common Pitfalls to Watch Out For
- Forgetting to count all the valence electrons in the structure.
- Misplacing atoms, which can mess up the molecule's representation.
- Overlooking the importance of electron sharing in covalent bonds, which is super important for molecular stability.
Methane's Role in Organic Chemistry
Methane is the building block of organic chemistry. It’s the simplest alkane and a major part of natural gas. Its stability and reactivity make it an essential molecule in many chemical reactions, including combustion and substitution reactions. In organic chemistry, methane's Lewis dot structure gives us valuable insights into how it behaves in different environments. For instance, its tetrahedral geometry plays a huge role in how it interacts with other molecules and why it’s so important in energy production.
Read also:Katiana Kay Erome The Star Who Keeps Shining
Even though methane’s structure seems simple, its importance in the chemical world is anything but. Its properties and behavior are the foundation for understanding more complex organic compounds. It’s a must-know topic for students and professionals alike. Think of it as the foundation for the skyscraper of organic chemistry!
How Methane Gets Used
- Energy source: Methane is a key part of natural gas, widely used for heating and generating electricity.
- Industrial applications: It’s used as a raw material for making hydrogen, methanol, and other essential chemicals.
- Environmental impact: Methane is a powerful greenhouse gas, significantly contributing to global warming and climate change.
Methane's Molecular Geometry and Bond Angles
The Methane Lewis Dot structure shows methane’s tetrahedral molecular geometry, where the carbon atom is at the center, and the four hydrogen atoms are equally spaced. This setup results in bond angles of about 109.5°, a hallmark of sp³ hybridized orbitals. Understanding methane's geometry is key to predicting its physical and chemical properties, like its boiling point, solubility, and how it reacts with other substances.
Methane's geometric arrangement isn’t just a theory—it has real-world implications in science and industry. By understanding the factors that shape methane's geometry, scientists can better predict and control how it behaves in different environments. It’s like having a cheat code for working with methane!
What Shapes Methane's Geometry?
- Electron repulsion: The push and pull between electron pairs is a big factor in determining the molecule's shape.
- Hybridization: Carbon's sp³ hybridization leads to the tetrahedral arrangement, making the molecule as stable and functional as possible.
- Environmental factors: External conditions can slightly tweak bond angles and geometry, affecting how the molecule behaves in specific situations.
Methane vs. Other Hydrocarbons
While methane is the simplest hydrocarbon, comparing it with molecules like ethane (C₂H₆) and propane (C₃H₈) highlights the importance of Lewis dot structures in understanding molecular bonding. Each hydrocarbon has a unique structure and set of properties, influenced by the number of carbon atoms and how they bond. For example, ethane's Lewis dot structure shows two carbon atoms connected by a single bond, with each carbon also bonded to three hydrogen atoms. This difference in structure leads to changes in reactivity and physical properties, showing how crucial molecular geometry is in chemistry.
How Methane Stands Apart
- Number of carbon atoms: Methane has one carbon atom, while ethane and propane have two and three, respectively.
- Bonding patterns: Methane shows simple tetrahedral geometry, whereas larger hydrocarbons have more complex structures, affecting their behavior and uses.
- Reactivity: Methane is generally less reactive compared to larger hydrocarbons due to its simpler structure, making it more stable in various conditions.
Methane's Environmental Footprint
Methane is a major greenhouse gas, with a global warming potential 28 times greater than carbon dioxide over a century. Its release into the atmosphere contributes to climate change, making it a critical focus for environmental scientists and policymakers. Understanding methane's Lewis dot structure and molecular properties helps researchers come up with strategies to reduce its environmental impact. For example, capturing methane emissions from landfills and agricultural activities can significantly cut down on its contribution to global warming, paving the way for a more sustainable future.
Ways to Cut Methane Emissions
- Improved agricultural practices: Using innovative techniques to reduce methane emissions from livestock and rice cultivation.
- Landfill management: Setting up systems to capture methane from decomposing organic waste and turning it into a renewable energy source.
- Energy efficiency: Reducing methane leaks during natural gas production and transportation through advanced technologies and protocols.
Wrapping It Up
So, there you have it! The Methane Lewis Dot structure gives us a foundational understanding of methane's molecular bonding and geometry. By studying this structure, we gain valuable insights into methane's properties, applications, and environmental impact. Whether you're diving into the details of organic chemistry or tackling the challenges of climate change, methane's Lewis dot structure is a vital tool for scientific discovery and innovation.
We’d love to hear your thoughts and questions in the comments section below. If you're keen to learn more, check out related topics like the Lewis dot structures of ethane and propane, or explore the broader implications of methane in environmental science. Together, let’s keep learning and work toward a more sustainable future!
Table of Contents
Article Recommendations